ISLAMABAD – The Drug Regulatory Authority of Pakistan (DRAP) has seized 11 fake medicines in various cities of Punjab, imposing ban on the sale and purchase of fake batches of medicine.
The action was taken after the Punjab Drug Testing Laboratory confirmed that these medicines are spurious, substandard of misbranded.
The fake medicines, which pose serious threat to lives of patients, were produced under the names of various brands of the leading pharmaceutical companies in the country.
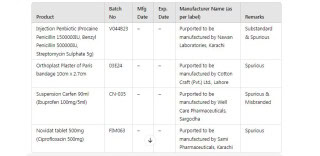
The identified fake products include Injection Penbiotic (Procaine Penicillin 1500000IU, Benzyl Penicillin 500000IU, Streptomycin Sulphate 5g) batch number V044B23. Following are some other counterfeit medicines detected by the authorities:
Injection Penbiotic (Procaine Penicillin 1500000IU, Benzyl Penicillin 500000IU, Streptomycin Sulphate 5g)
Orthoplast Plaster of Paris bandage 10cm x 2.7cm
Orthoplast Plaster of Paris bandage 10cm x 2.7cm
Suspension Carfen 90ml (Ibuprofen 100mg/5ml)
Novidat tablet 500mg (Ciprofloxacin 500mg)
“Adverse reactions or quality problems experienced with the use of these products are to be reported to the National Pharmacovigilance Centre (NPC), DRAP using Adverse Event Reporting Form or online through this link,” read the notice.
Moreover, DRAP has issued recall alerts for these products after receiving a request from the Punjab government.
Advise for Manufacturers
DRAP has issued an advise for manufacturers of therapeutic goods:
- Recall Products: If any batch was manufactured using the same lot of Propylene Glycol that has been identified as contaminated, all finished products from local and export markets should be recalled.
- Hold Other Batches: All finished products manufactured from the same lot of propylene glycol should be kept on hold. These products should be tested for EG/DEG contamination before releasing them into the supply chain.
- Screen Raw Materials: Before using them in the manufacturing of oral liquid preparations, all raw materials should be screened for contamination with EG and DEG.
- Compliance: Ensure compliance with all directives issued by DRAP to safeguard public health from contaminated products.
- Follow Guidelines: Adhere to the pharmacopoeia monograph and WHO guidelines for testing EG/DEG in oral liquid preparations during the analysis of both raw materials and finished products.










