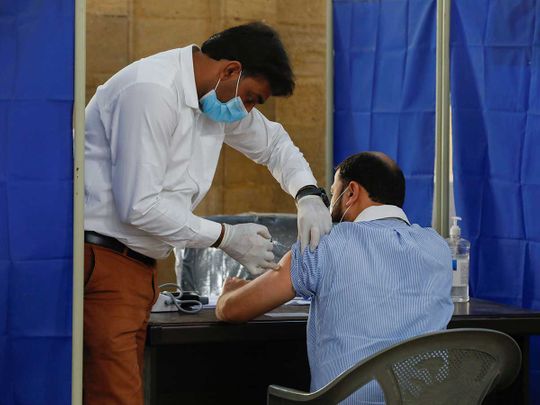
ISLAMABAD – Pakistan has placed an order to procure Chinese CanSino Biologics Inc.’s single-dose vaccine against COVID-19, it emerged on Wednesday.
An official of the National Health Serviced told a private media outlet that the first shipment of 60,000 doses of Ad5-nCoV vaccine is expected to be delivered within a week.
The official further said that the single-dose vaccine could be given to people of every age as it was found effective among people from 18 to 80 plus years of age during trials in Pakistan and other countries of the world.
He also hinted that the vaccine would be partially developed in Pakistan after successful trials of the medicine in the country, The News reported.
Currently, Pakistan is administering two-dose vaccine gifted by China to frontline health workers and elderly people.
In February, Pakistan had approved China’s CanSinoBio Covid-19 vaccine for emergency use.
“Yes, Correct,” Special Assistant to Prime Minister on Health Dr Faisal Sultan said after being asked to confirm that the Drug Regulatory Authority of Pakistan (DRAP) had met and approved the vaccine.
CanSinoBio had released interim efficacy results of a multi-country trial, which included Pakistan, showing 65.7% efficacy in preventing symptomatic coronavirus cases and a 90.98% success rate in stopping severe infections.
“Clinical trial data (phase-3) of a one-dose Ad5-nCoV vaccine for Covid (Cansino Bio) received. Interim analysis by the Independent Data Monitoring Committee shows 65.7% efficacy at preventing symptomatic cases.