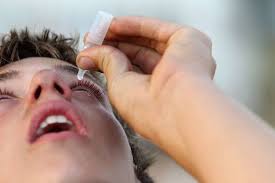
Media reported due to a probable Pseudomonas aeruginosa contamination, a type of bacteria that is typically drug-resistant, the manufacturer of eye drops sold under the name EzriCare withdrew them on Thursday.
In 12 US states, at least 55 bacterial illnesses have been linked to EzriCare artificial tears. So far, five of those people have had vision loss.
One individual died after the bacterium entered their circulation.
Global Pharma Healthcare, an Indian company, voluntarily recalled its Artificial Tears Lubricant Eye Drops on Thursday. The recall comes in response to a request for individuals to cease using the eye drops right away from the Centers for Disease Control and Prevention.
Global Pharma Healthcare stated in its recall notification that “the medicine was distributed nationally in the USA over the internet.”
The drops are distributed by EzriCare and Delsam Pharma. EzriCare asserted that it “had no role in the formulation, packaging delivery mechanism, or actual production of this drug” in a statement released on Wednesday. The company claimed that all it did was manufacture and sell the product’s label.
The Food and Drug Administration, state and local health officials, and other organisations will work together, the CDC announced on Wednesday, to investigate the problem. The majority of patients claimed to have used these eye drops prior to becoming ill.