A parliamentary committee in Gambia said on Tuesday that India-based drug maker Maiden Pharmaceuticals was responsible for the deaths of at least 70 children from acute kidney injury and called on the government to pursue legal action.
The World Health Organisation said in October four medicinal syrups made by Maiden and imported by a local wholesaler were likely linked to the deaths, which have shocked the West African country since July. The drugs were pulled from the shelves and Maiden’s production licence in India was suspended.
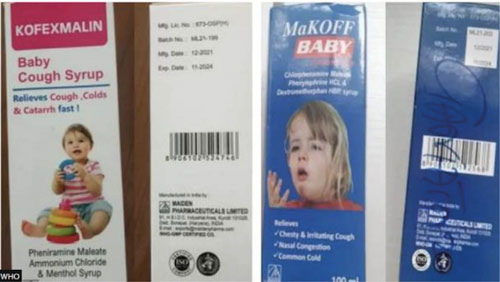
According to the WHO, lab analysis confirmed “unacceptable” amounts of diethylene glycol and ethylene glycol in the medicines made by Maiden.
But the global health body told the BBC it was only following its mandate and “stands by the action taken”.
After weeks of investigation, the Gambian par-liamentary committee has now recommended that authorities should take tough measures, including banning all Maiden Pharmaceuticals products in the country and taking legal action against the firm.
The committee said it “is convinced that Maiden Pharmaceuticals [is] culpable and should be held accountable for exporting the contaminated medi-cines”.
“The findings remain the same with the previous reports which indicates that Promethazine Oral So-lution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup and Magrip N Cold Syrup were contaminated with diethylene glycol and ethylene glycol,” the parliamentary committee said in its report.
Diethylene glycol and ethylene glycol are toxic to humans and could be fatal if consumed. But the panel added that the exact scientific cause of the children’s deaths was still under investigation.
The committee also wanted the country’s Medi-cine Control Agency to ensure all medicines imported into the country are properly registered and back-ground checks conducted on manufacturers – includ-ing visiting their facilities.
The report also revealed inadequacies in the country’s healthcare system urging the government to strengthen it and provide better equipment and medicines to the country’s hospitals.
In late July, The Gambia detected an increase in cases of acute kidney injury among children under the age of five. The government later said around 69 children had died from these injuries.—Agencies