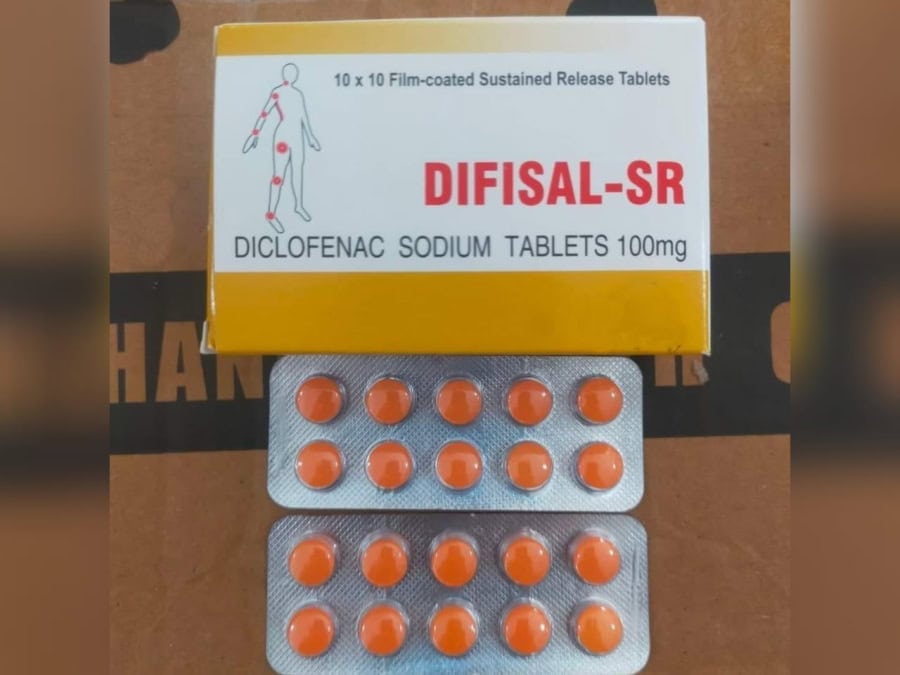
Presence of substandard and adulterated Tablet Difisal SR in the market has put the health and even the lives of gout, and arthritis patients at risk.
After Punjab Drug Testing Laboratory Faisalabad found samples of Tab Difisal SR ‘substandard and adulterated with Ciprofloxacin’, the government directed recalling batch 21L718 of this drug for saving patients from life-threatening complications.
The shocking revelations raise question how this frequently prescribed drug will be removed from retail outlets and wholesale markets across the country.
As per the physicians, people know little or nothing about a batch. Moreover, the quantity of drugs in the said batch has also not been mentioned.
“Recalling drug from the market is an uphill task. Patients have already consumed this drug. More and more will continue to take it as this drug is largely prescribed. I cannot understand how one batch has reached market before DTL approval”, said Dr Abdul Rauf, a family physician running a clinic in Usman Gunj, a densely populated locality in Northern Lahore.
He said that such incidents have taken hundreds of precious human lives in the past. He mentioned the past example of the dispensation of adulterated drugs to patients at the Punjab Institute of Cardiology.
“The drug had taken hundreds of lives before the government identified the real issue”, he said, adding that the government should make arrangements for immediate recall of the said batch and award exemplary punishment to responsible people.
Manufactured by M/S Iqra Pharmaceuticals, Tab Difisal SR (Diclofenac Sodium 100mg) is prescribed for relieving pain, stiffness, and swelling of the joints in conditions like rheumatoid arthritis, osteoarthritis, gout and ankylosing spondylitis.

This drug also helps in relieving pain following dental surgery, surgical procedures and minor injury.
The Punjab government has directed retailers (pharmacies, medical stores), wholesalers, distributors and health facilities to immediately stop the dispensation of the batch. It also directed updating respective area drug inspectors regarding current inventory and consumption record of the above-mentioned batch.
The government has advised consumers (patients, healthcare professionals) to stop the use of said batch immediately as it may be prone to risk.
“In case of adverse drug reactions or quality problems with the use of this product, must be reported to Provincial Pharmacovigilance Centre”, the advisory reads.