New York
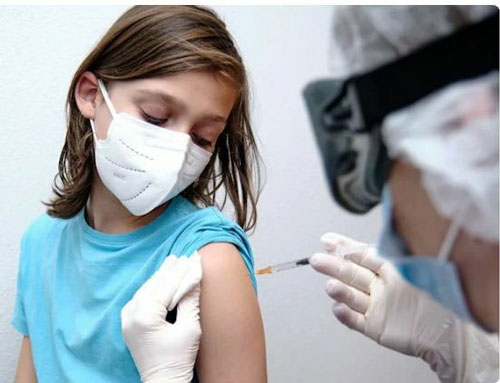
The Pfizer coronavirus vaccine has been approved for use in children aged 12 to 15 by regulators in the US in what was described as a “watershed moment” in the effort to bring the pandemic under control.
Until now, only those aged 16 and older have been permitted to receive the two-dose vaccine.
The Federal Drug Administration’s (FDA) approval after a weeks-long safety review must be ratified by an advisory committee of the government’s Centres for Disease Control and Prevention (CDC).
If it gives its go ahead at a scheduled meeting on Wednesday, vaccinations of children can begin immediately.
The authorisation comes after a successful wide-scale trial of the vaccine in which 2,260 volunteers aged 12 to 15 received two doses of either the vaccine or a placebo three weeks apart.
There were no cases of Covid-19 among those who received the vaccine, and 18 in those who received the dummy shot. Side effects were comparable to those seen in a similar trial of 16- to 25-year-olds, the FDA found.
“This is a watershed moment in our ability to fight back the Covid-19 pandemic,” said Dr Bill Gruber, a senior vice-president of Pfizer and a paediatric doctor.
“We have safety, we got the immune response we wanted, it was actually better than what we saw in the 16 to 25 population, we had outright demonstration of efficacy.”
Dr Richard Besser, a former acting director of the CDC, said the authorisation brought him “hope and joy”.
“There are now millions more who can be protected from Covid and a lot of hope that this fall school is going to feel a lot more like school should feel like,” he told CNN.
Trials continue in children for the Moderna vaccine, the only other two-dose vaccine already approved for adults in the US. Results are expected later in the summer.
The FDA said that its regulatory panel would meet again on June 10 to discuss a roadmap for authorising Pfizer vaccines for those under 12.—AP