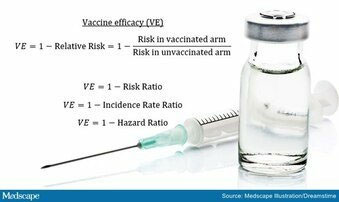
VACCINE efficacy is the percentage reduction in a disease in a group of people who received a vaccination in a clinical trial. It differs from vaccine effectiveness, which measures how well a vaccine works when given to people in the community outside of clinical trials.
All new vaccines undergo clinical trials to test how well they work. The developers of a vaccine candidate usually determine the main goals of their trial in their clinical trial study protocol.
These goals are called the primary endpoints. For many experimental Covid-19 vaccines currently in development, the primary endpoints focus on preventing new cases of symptomatic Covid-19.
Stay informed with live updates on the current Covid-19 outbreak and visit our coronavirus hub for more advice on prevention and treatment.
Scientists can calculate how well a vaccine candidate works by looking at the difference in new cases of the disease between the group receiving a placebo and the group receiving the experimental vaccine.
This is called vaccine efficacy. For example, Pfizer/BioNTech reported an efficacy of 95% for the Covid-19 vaccine. This means a 95% reduction in new cases of the disease in the vaccine group compared with the placebo group.
Volunteers taking part in vaccine clinical trials often undergo close monitoring. The trial team is usually aware of the participants’ general health and any relevant health conditions.
Participants usually report any side effects and may fill out daily symptom monitoring diaries.
Many clinical trials have exclusion criteria such as pregnancy, particular health conditions, and age. Trials involving experimental vaccines rarely include children or seniors until scientists have collected a significant amount of safety data to protect these groups from potential harm.
Vaccine efficacy only provides information about how well a vaccine works under the conditions of the clinical trial. Scientists usually base it on factors that they can quantify, such as numbers of laboratory-confirmed cases of Covid-19.
But the ideal conditions of a clinical trial do not necessarily reflect what is happening in the real world outside of clinical trials.
Vaccine effectiveness tells us how well a vaccine works under real-world conditions once people outside of clinical trials receive the vaccine.
Many factors can influence how a vaccine performs outside of clinical trials. One of these is the health of those receiving the vaccine. Underlying health conditions can affect vaccine effectiveness.
Another factor is how the disease-causing pathogen changes with time.