Staff Reporter
Islamabad
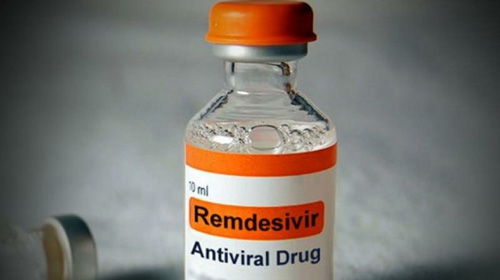
Drug Regulatory Authority of Pakistan (DRAP) has issued registration letters for import and manufacturing of remdesivir used for critically ill patients of COVID-19.
According to the Ministry of National Health Services, on increasing demand, approvals are issued to two importers and 14 local manufacturers.
The spokesperson said COVID-19 drug was approved for emergency use in a special emergency meeting in order to ensure early availability.
Special Assistant to the Prime Minister (SAPM) on Health Dr Zafar Mirza yesterday said the government is ensuring sufficient supplies of remdesivir for critical Covid-19 patients.
Separately, Dr Zafar Mirza in a tweet said the DRAP has completed “national stock taking” of dexamethasone – a steroid drug that has shown to be effective in the treatment of critical Covid-19 patients. “I am pleased to report that we have enough stocks of the medicine available in Pakistan. It is available on prescription only.